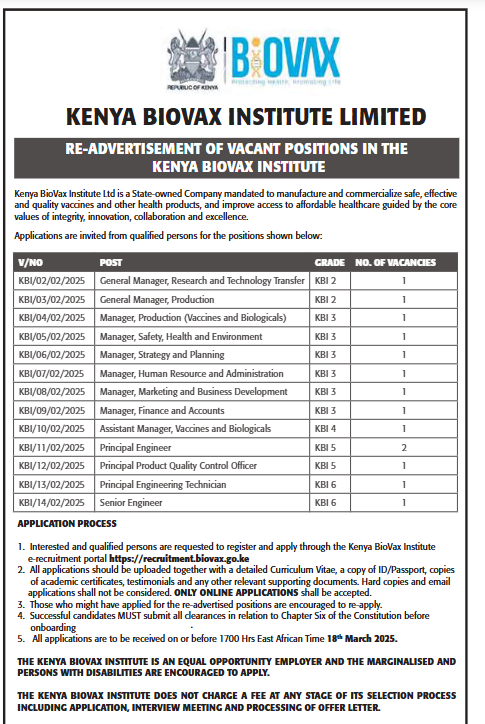
Kenya BioVax Institute Ltd is a State-owned Company mandated to manufacture and commercialize safe, effective, and quality vaccines and other health products. Our mission is to improve access to affordable healthcare guided by the core values of integrity, innovation, collaboration, and excellence.
As part of our continued commitment to excellence and innovation, we are seeking qualified professionals to fill various positions within our organization. These roles provide a unique opportunity to contribute to the growth of Kenya’s healthcare sector and make a tangible impact on public health.
We invite applications from suitably qualified individuals for the following positions:
General Manager, Research and Technology Transfer
Grade: KBI 2
No. of Vacancies: 1
Job Description:
The General Manager, Research and Technology Transfer, will be responsible for leading and overseeing the development of innovative healthcare solutions, including vaccines and biologics. The role involves identifying and implementing new technologies, establishing research partnerships, and ensuring that the Institute remains at the forefront of vaccine development. The incumbent will also be responsible for translating research into commercially viable products while ensuring compliance with industry standards.
General Manager, Production
Grade: KBI 2
No. of Vacancies: 1
Job Description:
The General Manager, Production, will be responsible for overseeing the entire production process of vaccines and other healthcare products. The role involves ensuring efficient manufacturing processes, maintaining high production quality, and meeting regulatory standards. The incumbent will work closely with research and quality control teams to optimize production methods and ensure continuous improvement.
Manager, Production (Vaccines and Biologicals)
Grade: KBI 3
No. of Vacancies: 1
Job Description:
The Manager, Production (Vaccines and Biologicals), will oversee daily production activities within the manufacturing unit. The role involves supervising production staff, ensuring compliance with Good Manufacturing Practices (GMP), monitoring process efficiency, and implementing quality control measures. The incumbent will also liaise with regulatory authorities to ensure adherence to safety and operational guidelines.
Manager, Safety, Health, and Environment
Grade: KBI 3
No. of Vacancies: 1
Job Description:
The Manager, Safety, Health, and Environment, will be responsible for developing and implementing safety policies to ensure a safe and compliant working environment. The role includes conducting risk assessments, overseeing health and safety training, and ensuring that all activities align with national and international environmental standards. The incumbent will also work to promote a culture of health and safety within the organization.
Manager, Strategy and Planning
Grade: KBI 3
No. of Vacancies: 1
Job Description:
The Manager, Strategy and Planning, will be responsible for developing and implementing strategic initiatives that align with the organization’s mission. The role includes conducting market research, analyzing industry trends, and developing business models to enhance the Institute’s competitiveness. The incumbent will also collaborate with stakeholders to ensure that strategic objectives are met efficiently.
Manager, Human Resource and Administration
Grade: KBI 3
No. of Vacancies: 1
Job Description:
The Manager, Human Resource and Administration, will be responsible for overseeing all human resource functions, including talent acquisition, employee relations, performance management, and compliance with labor laws. The role also involves developing and implementing HR policies, managing administrative functions, and fostering a positive organizational culture.
Manager, Marketing and Business Development
Grade: KBI 3
No. of Vacancies: 1
Job Description:
The Manager, Marketing and Business Development, will develop and execute strategies to promote Kenya BioVax Institute’s products and services. The role includes market analysis, customer engagement, brand management, and identifying new business opportunities. The incumbent will also work on strengthening partnerships with industry stakeholders to expand market reach.
Manager, Finance and Accounts
Grade: KBI 3
No. of Vacancies: 1
Job Description:
The Manager, Finance and Accounts, will oversee financial planning, budgeting, and financial reporting. The role involves ensuring financial integrity, managing cash flows, and ensuring compliance with financial regulations. The incumbent will also provide strategic financial guidance to support decision-making within the organization.
Assistant Manager, Vaccines and Biologicals
Grade: KBI 5
No. of Vacancies: 1
Job Description:
The Assistant Manager, Vaccines and Biologicals, will support the production and quality control teams in vaccine manufacturing. The role includes overseeing the formulation and packaging of vaccines, maintaining records, and ensuring compliance with industry regulations.
Principal Engineer
Grade: KBI 5
No. of Vacancies: 1
Job Description:
The Principal Engineer will be responsible for designing, implementing, and maintaining engineering systems within the Institute. The role involves overseeing technical projects, ensuring compliance with safety standards, and optimizing production processes through engineering solutions.
Principal Product Quality Control Officer
Grade: KBI 5
No. of Vacancies: 1
Job Description:
The Principal Product Quality Control Officer will ensure that all products meet quality and safety standards before they are released to the market. The role includes conducting quality tests, maintaining documentation, and ensuring compliance with regulatory requirements.
Principal Engineering Technician
Grade: KBI 5
No. of Vacancies: 1
Job Description:
The Principal Engineering Technician will support the engineering team in the maintenance and operation of technical equipment. The role involves troubleshooting mechanical and electrical issues, conducting routine inspections, and ensuring that all systems are functioning optimally.
Senior Engineer
Grade: KBI 5
No. of Vacancies: 1
Job Description:
The Senior Engineer will be responsible for managing engineering projects, overseeing maintenance operations, and ensuring the reliability of technical systems. The role involves collaborating with different departments to enhance efficiency and reduce downtime.
Application Process
Interested and qualified persons are requested to register and apply through the Kenya BioVax Institute e-recruitment portal at https://recruitment.biovax.go.ke
All applications should be uploaded together with a detailed Curriculum Vitae, a copy of ID/Passport, copies of academic and professional testimonials, and any other relevant supporting documents. Hard copies and email applications will not be accepted.
Shortlisted candidates will be contacted for further assessments.
All applications must be submitted on or before 1700 hrs East African Time, 18th March 2025.
Kenya BioVax Institute is an equal opportunity employer, and we encourage applications from marginalized groups and persons with disabilities.
The Kenya BioVax Institute does not charge a fee at any stage of its selection process, including application, interview meeting, and processing of offer letters